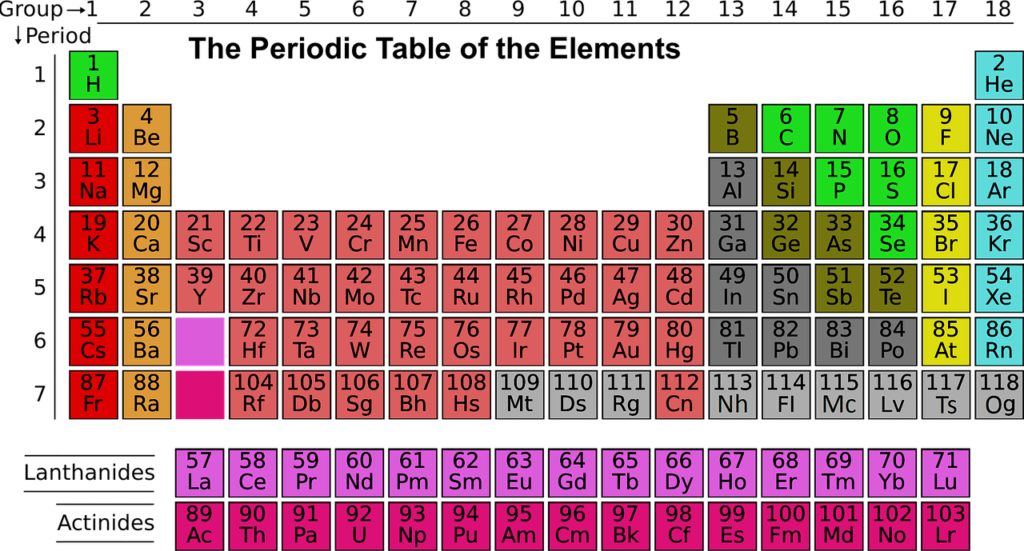
The modern periodic table has its elements listed in the order of their increasing atomic number. The atomic number is the number of protons that are present in the nucleus of the atom. It is the number of protons that defines the identity of the element.
So, if you say that an element has 6 protons, it refers only to the carbon atom. This is irrelevant to the number of neutrons it has.
The periodic table has its number arranged in the increasing order of its atomic number. The proton number determines the number of electrons that surround the nucleus. It is this electron arrangement that determines the elements’ chemical behaviour.
The elements that have the same chemical properties line up naturally in the same group. So, if you were to look at group 1A, these are soft metals that react with water violently and form a +1 charge.
On the other hand, if you look at group 8A, these are monoatomic and unreactive gases present at room temperature. So, you see that there is a sort of a periodic repetition of the properties of the chemical elements with its increase in mass.
The first 20 elements have a lot of significance, which will be discussed below.
The Periodic Table
Dimitri Mendeleev published the original periodic table in 1869, and the elements here were arranged based on its increasing atomic mass. This was when the nucleus was not discovered, and there was no clear understanding of the atoms’ interior structure.
Atomic mass was thus the only reference point. Once the nucleus structure got clear, the atomic number was used to govern the properties of the elements.
The periodic table organizes its elements in the increasing order of their atomic number, represented by the number of protons in the atom. The elements that are placed in the same column have the same properties and form a group. The elements placed in the same row are called periods, and these have the same energy levels of the electrons in the unexcited state.
The periodic table is used to find the usual charge and the atomic weight of the element. All of these are compiled into one reference table called the periodic table.
Initial 20 elements of the periodic table and its atomic number
Here are the first twenty elements of the periodic table that have been tabulated below.
| Atomic Number | Element | Symbol |
|---|---|---|
| 1 | Hydrogen | H |
| 2 | Helium | He |
| 3 | Lithium | Li |
| 4 | Beryllium | Be |
| 5 | Boron | B |
| 6 | Carbon | C |
| 7 | Nitrogen | N |
| 8 | Oxygen | O |
| 9 | Fluorine | F |
| 10 | Neon | Ne |
| 11 | Sodium | Na |
| 12 | Magnesium | Mg |
| 13 | Aluminium | Al |
| 14 | Silicon | Si |
| 15 | Phosphorus | P |
| 16 | Sulfur | S |
| 17 | Chlorine | Cl |
| 18 | Argon | Ar |
| 19 | Potassium | K |
| 20 | Calcium | Ca |
Each element has a distinct property determined by its atomic mass, atomic number, electronegativity, electronic configuration, enthalpy and electron gain, etc.
The noble gases that are a part of the first twenty periodic table elements
Noble gases or inert gases are located in the periodic tables 18th group. These are either non-reactive or are the least reactive. The characteristics of the noble gases were found out much later than the other periodic table elements.
These 20 elements consist of three noble gases which are helium, neon, and argon. The atomic number of helium is 2, the atomic number of neon is 10, and the atomic number of argon is 18.
Atomic mass and atomic number
To understand the periodic table better and figure out the characteristic of the elements, it is first essential to understand what the atomic mass and atomic number mean.
- Atomic mass: The particle of any matter, whether small or big, has some mass connected with it. Everything is built of atoms. The atomic mass refers to the mass of an atomic particle. This is stated as the AMU or the atomic mass unified number, which is as per the international agreement.
- Atomic number: Atomic number denotes the number of protons present in the atom’s nucleus. It is also the measure of the number of electrons present in an electrically neutral atom. This is the atomic number.
Figuring the importance of atomic numbers
The periodic table has been designed with the atomic number of the elements. The atomic number gives an insight into the number of protons present in the nuclei of the atoms and the number of electrons that surround this nucleus.
So if the atomic number of Sodium is 11, then it means that the sodium nucleus contains 11 protons and is surrounded by 11 electrons. Since the atomic number is the same as the elements’ protons, you can figure out the electronic configuration by knowing the atomic number.
The neutron of the atom cannot be determined based on its atomic number. This is because the elements could have different isotopes, which could have a similar number of electrons and protons but a different number of neutrons. So in the above example, sodium will always have 11 electrons and 11 protons, but its atom could have 11, 12 or 13 neutrons.
Figuring out the importance of the mass number
The mass number is denoted by A. When you add the neutrons and protons of an atom, this is the element’s mass number. The neutron and proton together are called the nucleons because both are present in the atom’s nucleus.
So Oxygen which has 8 protons and 8 neutrons, has a mass number of 16.
The mass of the electrons is almost negligible, and thus the atomic mass of an element is equal to the mass number. The atomic mass is calculated as the atomic mass unit. This is also called the Dalton, which is denoted by a D.
Conclusion
An element is a substance that cannot be further broken down into another substance through chemical means. It cannot also be transformed into any other substance. The atomic number signifies the number of protons present in the nucleus. It is a significant number that helps identify the properties of the elements that form the periodic table.